Solids Liquids And Gases

Solids, liquids, and gases are the three fundamental states of matter that have distinct physical properties and characteristics. Understanding these states is crucial in various fields, including physics, chemistry, and engineering. In this article, we will delve into the world of solids, liquids, and gases, exploring their definitions, properties, and behaviors.
Introduction to States of Matter

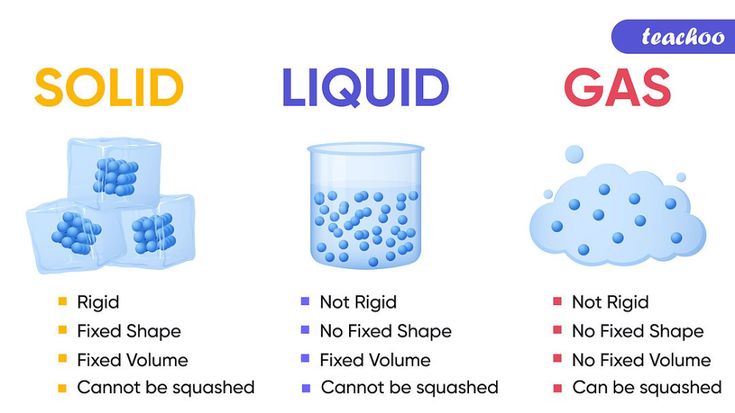
The state of matter is defined by the arrangement and motion of particles, such as atoms or molecules. The three main states of matter are solids, liquids, and gases, which are characterized by their unique properties and behaviors. Solids have a fixed shape and volume, liquids take the shape of their container and have a fixed volume, and gases have neither a fixed shape nor a fixed volume.
Properties of Solids
Solids have a crystalline structure, where particles are arranged in a repeating pattern. This arrangement gives solids their rigidity and resistance to deformation. Solids also have a fixed volume and shape, which are determined by the arrangement of their particles. Some common properties of solids include:
- High density
- Low compressibility
- High viscosity
- Ability to maintain shape and volume
Examples of solids include metals, such as iron and copper, and non-metals, such as wood and glass.
Properties of Liquids
Liquids have a more random arrangement of particles compared to solids, which allows them to flow and take the shape of their container. Liquids have a fixed volume but not a fixed shape. Some common properties of liquids include:
- Lower density than solids
- Higher compressibility than solids
- Lower viscosity than solids
- Ability to flow and take the shape of their container
Examples of liquids include water, oil, and gasoline.
Properties of Gases
Gases have a highly random arrangement of particles, which allows them to expand and fill their container. Gases have neither a fixed shape nor a fixed volume. Some common properties of gases include:
- Low density
- High compressibility
- Low viscosity
- Ability to expand and fill their container
Examples of gases include air, helium, and oxygen.
Phase Transitions

Phase transitions occur when a substance changes from one state of matter to another. There are several types of phase transitions, including:
Melting and Freezing
Melting occurs when a solid changes to a liquid, while freezing occurs when a liquid changes to a solid. The melting and freezing points of a substance are determined by its chemical composition and pressure.
Vaporization and Condensation
Vaporization occurs when a liquid changes to a gas, while condensation occurs when a gas changes to a liquid. The vaporization and condensation points of a substance are also determined by its chemical composition and pressure.
Sublimation and Deposition
Sublimation occurs when a solid changes directly to a gas, while deposition occurs when a gas changes directly to a solid. These phase transitions are less common and typically occur under specific conditions.
| Phase Transition | Description |
|---|---|
| Melting | Solid to liquid |
| Freezing | Liquid to solid |
| Vaporization | Liquid to gas |
| Condensation | Gas to liquid |
| Sublimation | Solid to gas |
| Deposition | Gas to solid |
Real-World Applications
The understanding of solids, liquids, and gases has numerous real-world applications. For example:
Refrigeration and Air Conditioning
Refrigeration and air conditioning systems rely on the principles of phase transitions to cool and heat buildings and vehicles. The systems use a refrigerant that changes state from liquid to gas and back to liquid, allowing heat to be transferred from one location to another.
Chemical Processing
Chemical processing plants use various phase transitions to manufacture products, such as plastics, fertilizers, and pharmaceuticals. The plants often involve the conversion of raw materials from one state of matter to another, such as from solid to liquid or from liquid to gas.
Energy Generation
Energy generation systems, such as power plants, rely on the principles of thermodynamics to convert energy from one form to another. The systems often involve the use of solids, liquids, and gases to generate electricity, such as in the case of steam turbines.
What is the difference between a solid and a liquid?
+A solid has a fixed shape and volume, while a liquid takes the shape of its container and has a fixed volume.
What is the process of a solid changing directly to a gas called?
+The process of a solid changing directly to a gas is called sublimation.
What is the importance of understanding phase transitions in industrial applications?
+Understanding phase transitions is crucial in various industrial applications, such as refrigeration, air conditioning, and chemical processing, as it allows for the efficient and safe operation of systems and equipment.