Ph How To Calculate: Easy Steps To Determine Levels

Calculating pH levels is a crucial aspect of understanding the chemical properties of a solution. pH, which stands for "potential of hydrogen," is a measure of the concentration of hydrogen ions in a solution. In this article, we will delve into the easy steps to determine pH levels, providing a comprehensive guide for both beginners and experts in the field.
Understanding pH Scale
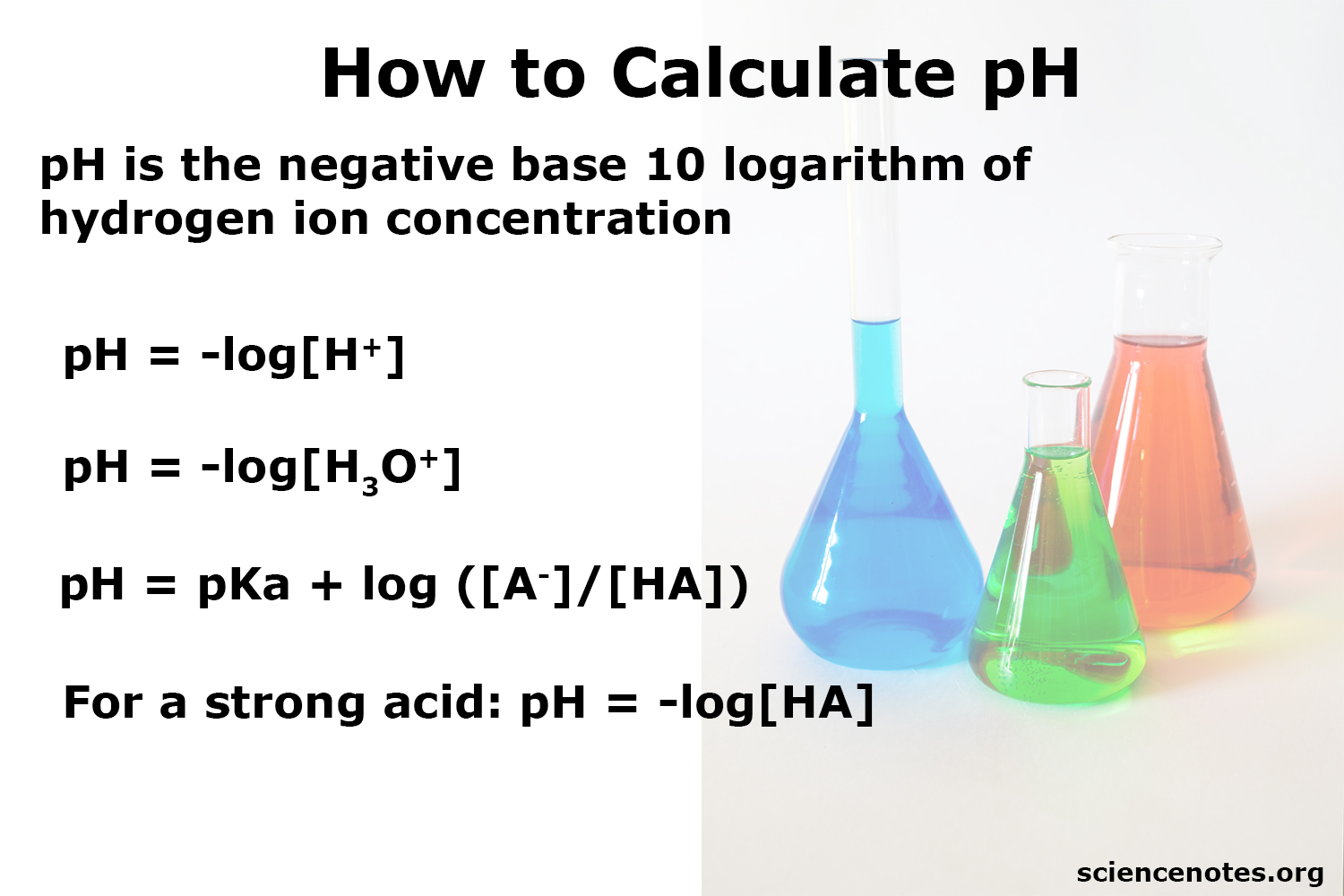
The pH scale is a logarithmic scale that ranges from 0 to 14, with 7 being the neutral point. A pH of less than 7 is considered acidic, while a pH of more than 7 is considered basic or alkaline. The pH scale is a fundamental concept in chemistry, and understanding it is essential for calculating pH levels. The pH scale is not linear, but rather logarithmic, meaning that each step up or down the scale represents a tenfold change in the concentration of hydrogen ions.
pH Calculation Formula
The pH of a solution can be calculated using the formula: pH = -log[H+], where [H+] is the concentration of hydrogen ions in moles per liter (M). This formula is based on the Nernst equation, which relates the electrode potential of a cell to the concentration of ions in the solution. To calculate pH, you need to know the concentration of hydrogen ions in the solution, which can be determined using various methods, such as titration or pH meter calibration.
| Concentration of Hydrogen Ions (M) | pH Value |
|---|---|
| 1 x 10^(-1) | 1 |
| 1 x 10^(-2) | 2 |
| 1 x 10^(-3) | 3 |
| 1 x 10^(-4) | 4 |
| 1 x 10^(-5) | 5 |
| 1 x 10^(-6) | 6 |
| 1 x 10^(-7) | 7 |
| 1 x 10^(-8) | 8 |
| 1 x 10^(-9) | 9 |
| 1 x 10^(-10) | 10 |
| 1 x 10^(-11) | 11 |
| 1 x 10^(-12) | 12 |
| 1 x 10^(-13) | 13 |
| 1 x 10^(-14) | 14 |

Easy Steps to Determine pH Levels

Now that we’ve covered the basics of pH calculation, let’s dive into the easy steps to determine pH levels:
- Prepare the solution: Prepare the solution by dissolving the substance in water or another solvent. Make sure to use a clean and dry container to avoid contamination.
- Calibrate the pH meter: Calibrate the pH meter according to the manufacturer's instructions. This ensures that the pH meter is accurate and reliable.
- Measure the pH: Dip the pH meter into the solution and take a reading. Make sure to stir the solution gently to ensure that the pH meter is in contact with the solution.
- Record the pH value: Record the pH value and make sure to note the temperature of the solution, as pH can be affected by temperature.
- Analyze the results: Analyze the results to determine if the solution is acidic, basic, or neutral. You can use the pH scale to determine the acidity or basicity of the solution.
Troubleshooting Common Issues
When calculating pH levels, you may encounter some common issues, such as:
- Incorrect calibration: Make sure to calibrate the pH meter regularly to avoid inaccurate results.
- Contamination: Use a clean and dry container to avoid contamination, which can affect the accuracy of the pH reading.
- Temperature fluctuations: Make sure to note the temperature of the solution, as pH can be affected by temperature fluctuations.
What is the pH of pure water?
+The pH of pure water is 7, which is neutral.
How do I calibrate a pH meter?
+To calibrate a pH meter, follow the manufacturer’s instructions, which usually involve immersing the electrode in a buffer solution of known pH.
What is the significance of pH in everyday life?
+pH plays a crucial role in everyday life, from determining the quality of drinking water to affecting the growth of plants and animals. It’s essential to understand pH to make informed decisions in various fields, including chemistry, biology, and environmental science.

