Lewis Structure Guide: Create Accurate Diagrams Fast
Understanding the structure of molecules is crucial in chemistry, and one of the most effective ways to visualize and understand molecular structure is through Lewis structures. Also known as electron dot diagrams, Lewis structures provide a simple yet powerful tool for representing the covalent bonds between atoms in a molecule. In this comprehensive guide, we will delve into the world of Lewis structures, explaining what they are, their importance, and most importantly, how to create them accurately and efficiently.
Introduction to Lewis Structures

Lewis structures are named after Gilbert N. Lewis, who introduced them in his 1916 article “The Atom and the Molecule.” These structures are a fundamental concept in chemistry, used to represent the arrangement of electrons in a molecule. They are particularly useful for understanding the covalent bonds between atoms, which are formed when atoms share one or more pairs of electrons to achieve a more stable electronic configuration.
Why Lewis Structures Matter
Lewis structures are essential for several reasons. Firstly, they help in understanding the molecular geometry and polarity, which are critical in predicting the physical and chemical properties of a substance. Secondly, Lewis structures are indispensable in organic chemistry for understanding reaction mechanisms and the stability of intermediates. Lastly, they provide a visual representation of the molecule, making it easier to understand complex chemical concepts.
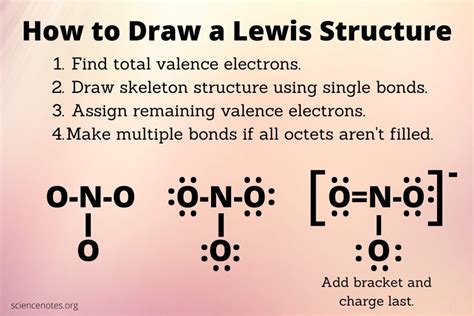
How to Draw Lewis Structures

Drawing Lewis structures involves several steps, which, when followed carefully, ensure that the resulting diagram accurately represents the molecule in question.
Step 1: Determine the Total Number of Valence Electrons
The first step in drawing a Lewis structure is to calculate the total number of valence electrons in the molecule. This is done by summing the valence electrons of each atom in the molecule. For main group elements, the number of valence electrons can be determined from the group number in the periodic table.
Step 2: Determine the Central Atom
Next, identify the central atom(s) in the molecule. Typically, the least electronegative atom is chosen as the central atom, around which the other atoms are arranged.
Step 3: Arrange the Atoms
Arrange the atoms around the central atom, usually with the more electronegative atoms on the outside. This is a general guideline and can be influenced by the specific chemistry of the molecule.
Step 4: Draw Single Bonds
Draw single bonds between the central atom and the surrounding atoms. Each single bond represents two shared electrons.
Step 5: Complete the Octet
For main group elements, the goal is to achieve a full outer energy level, often referred to as an octet (eight electrons in the outermost shell). Distribute the remaining valence electrons to achieve a full octet for each atom, except for hydrogen, which needs only two electrons.
Step 6: Check for Multiple Bonds
If, after completing the octet, there are still electrons left, they may form multiple bonds (double or triple bonds) between atoms. This usually occurs when there are not enough electrons to achieve an octet around every atom with single bonds alone.
Here is a simple table to summarize the steps for drawing Lewis structures:
| Step | Description |
|---|---|
| 1 | Determine the total number of valence electrons |
| 2 | Determine the central atom |
| 3 | Arrange the atoms |
| 4 | Draw single bonds |
| 5 | Complete the octet |
| 6 | Check for multiple bonds |

Common Challenges and Solutions
Despite the straightforward steps, drawing Lewis structures can sometimes be challenging, especially with complex molecules. A common issue is dealing with molecules that have more than one possible Lewis structure, known as resonance structures. These structures are particularly important in understanding the stability and reactivity of molecules.
Resonance Structures
Resonance occurs when a molecule can be represented by more than one Lewis structure that differ only in the arrangement of their electrons, without changing the positions of the atoms. The actual structure of the molecule is a hybrid of these resonance structures, and understanding resonance is critical for predicting molecular properties and reactivity.
Conclusion and Future Implications
In conclusion, Lewis structures are a powerful tool for understanding the covalent bonds in molecules and predicting their properties and behaviors. By mastering the steps to draw accurate Lewis structures, chemists can gain deep insights into molecular chemistry, facilitating advancements in fields from drug discovery to materials science. As chemistry continues to evolve, the importance of Lewis structures will endure, serving as a foundational element in the study of molecular interactions and chemical reactions.
What are Lewis structures used for in chemistry?
+Lewis structures are used to represent the arrangement of electrons in a molecule, helping in understanding molecular geometry, polarity, and reactivity. They are essential in organic chemistry for predicting reaction mechanisms and the stability of intermediates.
How do you determine the central atom in a Lewis structure?
+The central atom is typically the least electronegative atom in the molecule. This atom is chosen because it can more easily form bonds with other atoms, allowing for the distribution of electrons that achieves a full outer energy level for as many atoms as possible.
What are resonance structures, and why are they important?
+Resonance structures are Lewis structures that differ only in the arrangement of electrons, without changing the positions of the atoms. They are important because they represent a single, delocalized electronic state, which is crucial for predicting molecular properties and reactivity. Understanding resonance structures helps chemists understand why certain molecules exhibit specific behaviors under different conditions.



