How To Find Ksp
The solubility product constant, denoted as Ksp, is a measure of the equilibrium between a solid ionic compound and its ions in a solution. It is a fundamental concept in chemistry, particularly in the study of solubility and precipitation reactions. In this article, we will delve into the world of Ksp, exploring its definition, importance, and methods for finding it.
Definition and Importance of Ksp
Ksp is defined as the equilibrium constant for a solid ionic compound dissolving in a solution. It represents the concentrations of the ions in the solution at equilibrium. The Ksp value is a measure of the solubility of the compound, with higher values indicating greater solubility. Ksp is essential in various chemical applications, such as predicting the formation of precipitates, determining the solubility of compounds, and understanding the behavior of ions in solution.
Methods for Finding Ksp
There are several methods for finding Ksp, including:
- Direct Measurement: One of the most common methods for finding Ksp is by directly measuring the concentrations of the ions in the solution using techniques such as titration, spectroscopy, or chromatography.
- Solubility Measurements: Ksp can also be found by measuring the solubility of the compound in a solution. This involves dissolving a known amount of the compound in a solution and then measuring the concentration of the ions.
- Equilibrium Constant Expressions: Ksp can be calculated using equilibrium constant expressions, which involve the concentrations of the ions and the solubility of the compound.
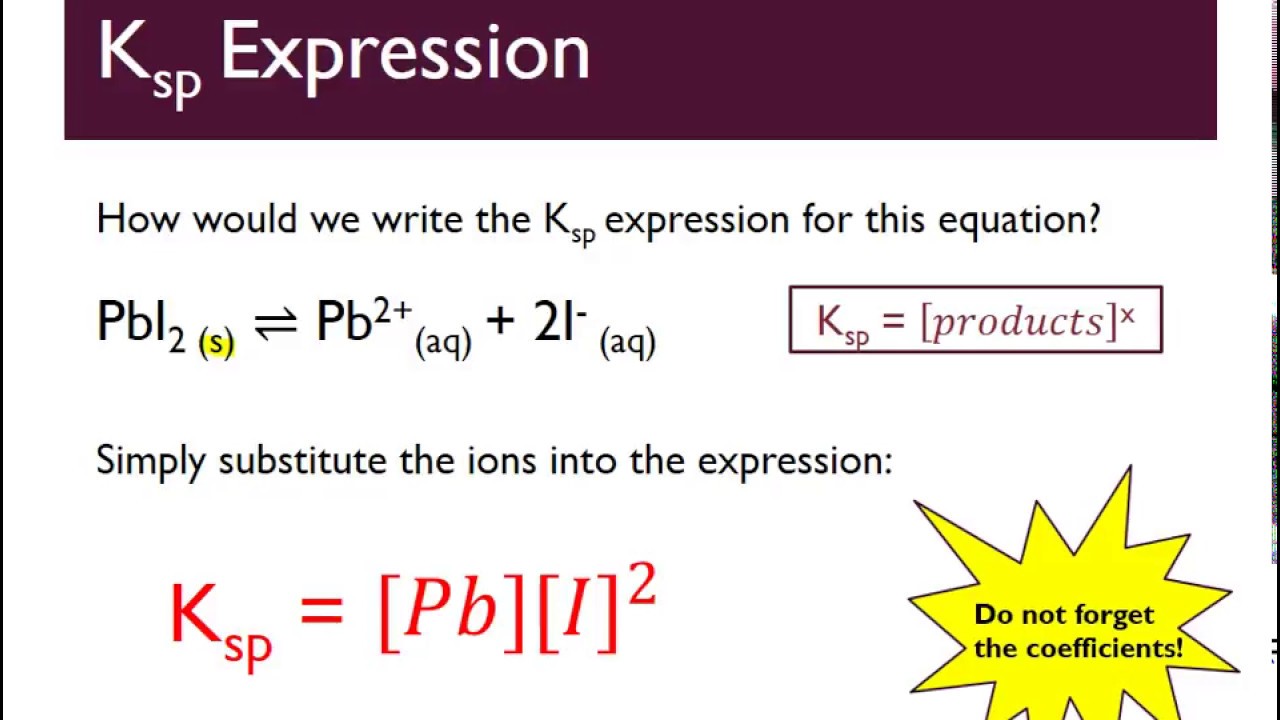
The Ksp expression is given by the equation: Ksp = [A]^a [B]^b, where [A] and [B] are the concentrations of the ions, and a and b are their respective stoichiometric coefficients.
Calculating Ksp
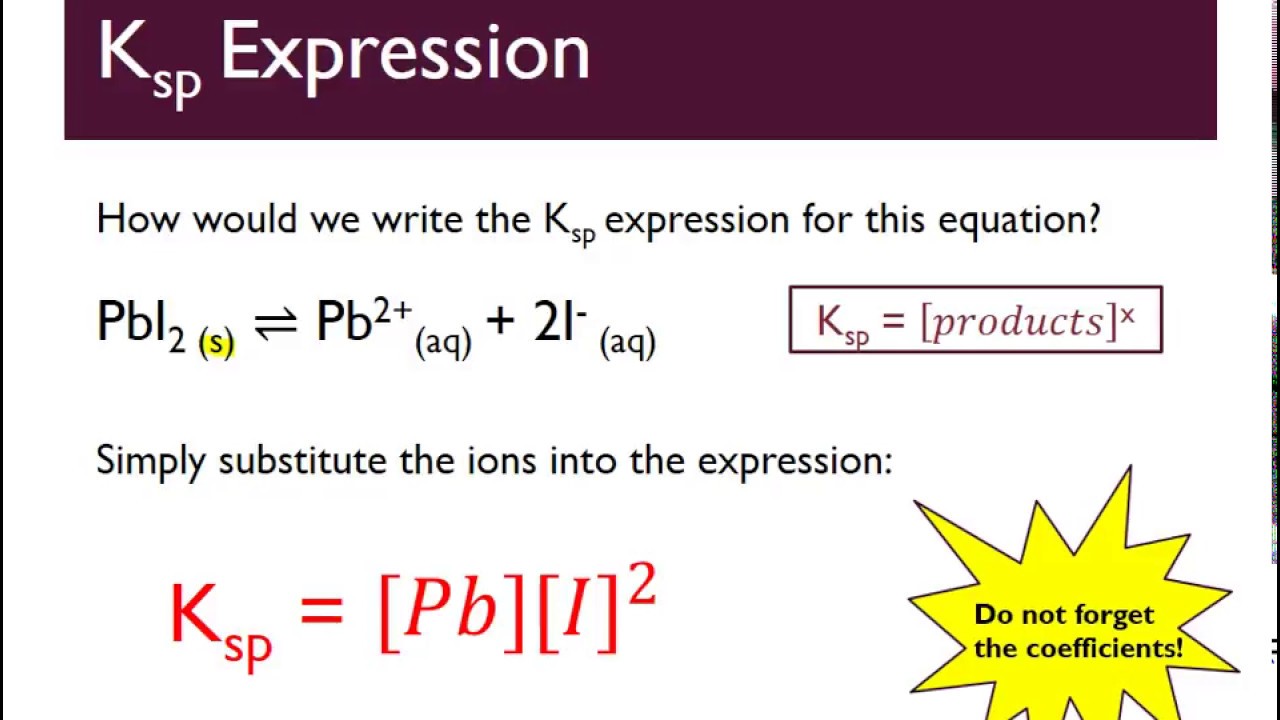
Once the concentrations of the ions are known, Ksp can be calculated using the equilibrium constant expression. For example, consider the dissolution of calcium carbonate (CaCO3) in water:
CaCO3 (s) ⇌ Ca2+ (aq) + CO32- (aq)
The Ksp expression for this reaction is: Ksp = [Ca2+] [CO32-]. If the concentrations of Ca2+ and CO32- are 0.01 M and 0.02 M, respectively, the Ksp value can be calculated as:
Ksp = (0.01 M) (0.02 M) = 2.0 x 10^-4 M^2
Factors Affecting Ksp
Ksp is affected by several factors, including temperature, pressure, and the presence of other ions in the solution. An increase in temperature generally increases the Ksp value, while an increase in pressure decreases it. The presence of other ions in the solution can also affect Ksp, particularly if they form complexes with the ions of the compound.
The following table illustrates the Ksp values for some common compounds:
| Compound | Ksp Value (M^2) |
|---|---|
| Calcium carbonate (CaCO3) | 8.9 x 10^-9 |
| Silver chloride (AgCl) | 1.8 x 10^-10 |
| Copper(II) sulfate (CuSO4) | 1.3 x 10^-5 |

Applications of Ksp
Ksp has numerous applications in chemistry, including:
- Predicting Precipitation Reactions: Ksp can be used to predict whether a precipitation reaction will occur when two solutions are mixed.
- Determining Solubility: Ksp can be used to determine the solubility of a compound in a solution.
- Understanding Ion Behavior: Ksp can help understand the behavior of ions in solution, including their interactions and complexation reactions.
In conclusion, Ksp is a fundamental concept in chemistry that plays a crucial role in understanding the behavior of ions in solution. By understanding how to find and calculate Ksp, chemists can better predict and control chemical reactions, which is essential in various fields, including environmental science, materials science, and pharmaceuticals.
What is the significance of Ksp in chemistry?
+
Ksp is significant in chemistry because it helps predict the formation of precipitates, determines the solubility of compounds, and understands the behavior of ions in solution.
How is Ksp affected by temperature and pressure?
+
Ksp is affected by temperature and pressure, with an increase in temperature generally increasing the Ksp value and an increase in pressure decreasing it.
What are some common applications of Ksp?
+
Ksp has numerous applications, including predicting precipitation reactions, determining solubility, and understanding ion behavior in solution.