Covalent Bond Definition: Understand Chemistry Basics
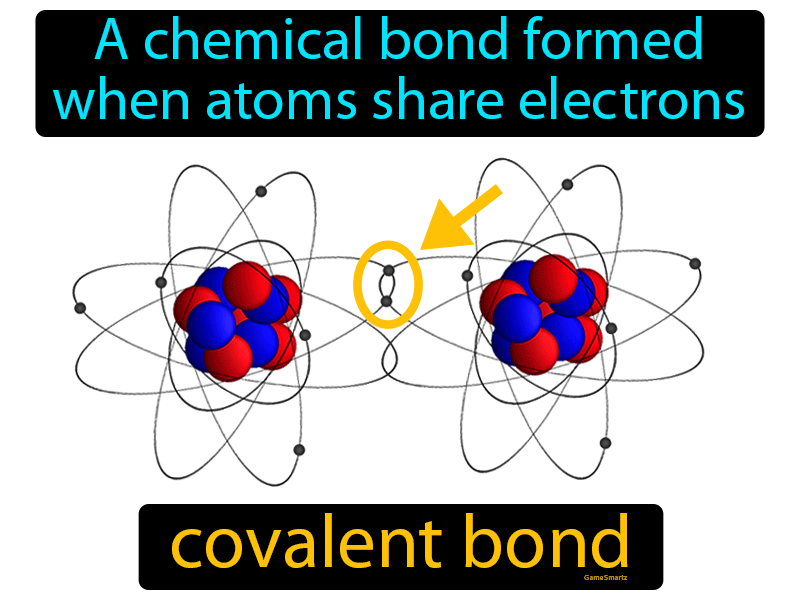
Covalent bonds are a fundamental concept in chemistry, representing a type of chemical bond where two or more atoms share one or more pairs of electrons to form a stable molecule. This definition is crucial in understanding the basics of chemistry, as covalent bonds are the primary means by which atoms form connections with each other. The sharing of electrons between atoms leads to the formation of a strong and stable bond, which is essential for the creation of molecules. In this article, we will delve into the definition of covalent bonds, their characteristics, and the different types of covalent bonds that exist.
Introduction to Covalent Bonds

A covalent bond is formed when two atoms share one or more pairs of electrons in order to achieve a full outer energy level. This sharing of electrons leads to a reduction in energy, making the molecule more stable. Covalent bonds can be formed between atoms of the same element, such as in the case of oxygen (O2) or nitrogen (N2), or between atoms of different elements, such as in the case of water (H2O) or carbon dioxide (CO2). The characteristics of covalent bonds include their strength, directionality, and polarity.
Characteristics of Covalent Bonds
Covalent bonds have several key characteristics that distinguish them from other types of chemical bonds. Strength is one of the primary characteristics of covalent bonds, with the bond strength depending on the number of electron pairs shared between the atoms. Polarity is another important characteristic, referring to the distribution of electrons within the bond. In polar covalent bonds, the electrons are not shared equally between the atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other. Directionality is also a key characteristic, as covalent bonds have a specific direction in space, which is determined by the orientation of the electron pairs.
| Type of Covalent Bond | Description |
|---|---|
| Nonpolar Covalent Bond | A covalent bond in which the electrons are shared equally between the atoms. |
| Polar Covalent Bond | A covalent bond in which the electrons are not shared equally between the atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other. |
| Coordinate Covalent Bond | A covalent bond in which one atom donates both electrons to the bond, while the other atom accepts them. |

Types of Covalent Bonds

There are several types of covalent bonds, including nonpolar covalent bonds, polar covalent bonds, and coordinate covalent bonds. Nonpolar covalent bonds are formed when the electrons are shared equally between the atoms, resulting in a symmetrical distribution of electrons. Polar covalent bonds are formed when the electrons are not shared equally between the atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other. Coordinate covalent bonds are formed when one atom donates both electrons to the bond, while the other atom accepts them.
Examples of Covalent Bonds
Covalent bonds are found in a wide range of molecules, from simple diatomic molecules such as oxygen (O2) and nitrogen (N2) to complex biological molecules such as proteins and DNA. In the case of water (H2O), the covalent bonds between the hydrogen and oxygen atoms are polar, resulting in a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. In the case of carbon dioxide (CO2), the covalent bonds between the carbon and oxygen atoms are nonpolar, resulting in a symmetrical distribution of electrons.
What is the definition of a covalent bond?
+A covalent bond is a type of chemical bond where two or more atoms share one or more pairs of electrons to form a stable molecule.
What are the characteristics of covalent bonds?
+The characteristics of covalent bonds include their strength, directionality, and polarity.
What are the different types of covalent bonds?
+The different types of covalent bonds include nonpolar covalent bonds, polar covalent bonds, and coordinate covalent bonds.