10 Heat Constant Facts For Better Understanding
The concept of heat constants is crucial in understanding various physical and chemical processes. Heat constants, such as the specific heat capacity and the heat of fusion, play a significant role in determining how substances respond to temperature changes. In this article, we will delve into 10 key facts about heat constants that are essential for a better understanding of thermodynamics and its applications.
Introduction to Heat Constants
Heat constants are numerical values that describe the thermal properties of substances. These constants are vital in predicting the behavior of materials under different temperature conditions. For instance, the specific heat capacity of a substance is a measure of the amount of heat energy required to raise the temperature of a unit mass of the substance by one degree Celsius. Understanding heat constants is essential in fields such as engineering, chemistry, and physics, where thermal management and energy efficiency are critical.
Specific Heat Capacity
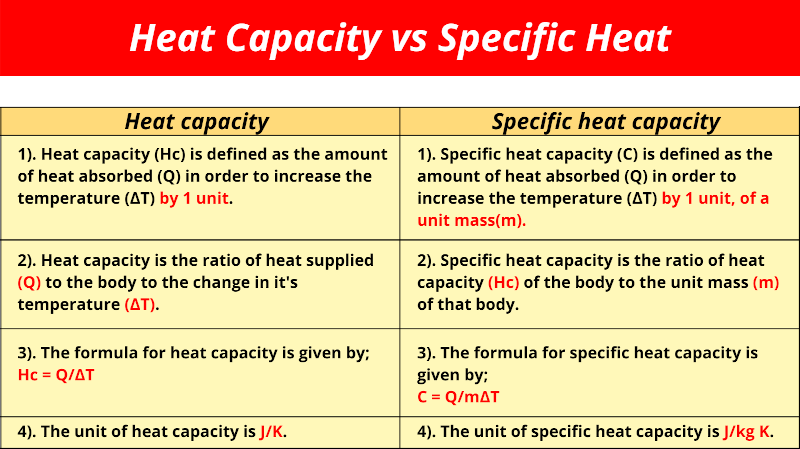
The specific heat capacity of a substance is a fundamental heat constant that characterizes its thermal properties. It is defined as the ratio of the amount of heat transferred to a substance to the resulting change in temperature. The specific heat capacity is typically denoted by the symbol ‘c’ and is measured in units of joules per kilogram per degree Celsius (J/kg°C). For example, the specific heat capacity of water is approximately 4.184 J/g°C, which means that it takes 4.184 joules of energy to raise the temperature of one gram of water by one degree Celsius.
| Substance | Specific Heat Capacity (J/g°C) |
|---|---|
| Water | 4.184 |
| Copper | 0.385 |
| Air | 1.005 |
Heat of Fusion and Vaporization

The heat of fusion and vaporization are other essential heat constants that describe the energy required to change the state of a substance. The heat of fusion is the energy required to melt a substance, while the heat of vaporization is the energy required to vaporize a substance. These constants are crucial in understanding phase transitions and are used in various applications, such as refrigeration, air conditioning, and thermal energy storage.
Latent Heat
Latent heat is the energy required to change the state of a substance without changing its temperature. The latent heat of fusion and latent heat of vaporization are measures of the energy required to melt and vaporize a substance, respectively. For example, the latent heat of fusion of ice is approximately 334 J/g, which means that it takes 334 joules of energy to melt one gram of ice at 0°C.
The understanding of heat constants, such as specific heat capacity, heat of fusion, and heat of vaporization, is vital in designing and optimizing various systems and applications. By grasping these fundamental concepts, engineers and scientists can develop more efficient and effective solutions for thermal management, energy storage, and conversion.
Real-World Applications
Heat constants have numerous real-world applications in various fields, including engineering, chemistry, and physics. For instance, the specific heat capacity of a substance is used in the design of heat exchangers, which are critical components in power plants, refrigeration systems, and air conditioning systems. The heat of fusion and vaporization are used in the development of thermal energy storage systems, which can store energy in the form of latent heat and release it when needed.
In addition to these applications, heat constants are also used in the development of phase change materials, which can absorb and release large amounts of energy during phase transitions. These materials have the potential to revolutionize the way we manage thermal energy and can be used in a wide range of applications, from building insulation to electronic cooling.
Future Implications
The study of heat constants has significant implications for the future of energy production, storage, and conversion. As the world transitions towards more sustainable and efficient energy solutions, the understanding of thermal properties and heat constants will play a critical role in the development of new technologies and applications. For instance, the development of advanced thermal energy storage systems could enable the widespread adoption of renewable energy sources, such as solar and wind power, by providing a reliable and efficient means of storing energy for later use.
In conclusion, heat constants are fundamental properties that describe the thermal behavior of substances. Understanding these constants is essential for the development of efficient and sustainable energy solutions, as well as for the design and optimization of various systems and applications. By grasping the concepts of specific heat capacity, heat of fusion, and heat of vaporization, engineers and scientists can unlock new technologies and innovations that will shape the future of energy production, storage, and conversion.
What is the specific heat capacity of water?
+
The specific heat capacity of water is approximately 4.184 J/g°C.
What is the latent heat of fusion of ice?
+
The latent heat of fusion of ice is approximately 334 J/g.
What are some real-world applications of heat constants?
+
Heat constants have numerous real-world applications, including the design of heat exchangers, thermal energy storage systems, and phase change materials.

